Key Knowledge:
|
The third line of defence against infectious disease is the adaptive immune system, which has two key qualities:
- It is specific (it can differentiate between specific microorganisms and respond accordingly)
- It is adaptive (it can produce a heightened response upon re-exposure - in other words, it has memory)
The adaptive immune system is slower to mobilise (compared to innate responses) and requires the activation of B and T lymphocytes
Antigen Presentation
The adaptive immune system becomes activated when specific pathogen fragments called antigens are presented to the lymphocytes
- T Lymphocytes cannot independently recognise antigens – they need to be introduced to the fragments by antigen presenting cells
- The antigenic fragment is complexed to an identification marker called an MHC molecule (major histocompatability complex molecule)
- There are two main types of MHC markers involved in antigen presentation (denoted as either class I markers or class II markers)
Class I markers are present on all nucleated body cells, allowing any cell that is compromised to act as an ‘amateur' presenting cell
- Class I markers present endogenous antigens (found internally) to cytotoxic T cells, which initiate a cell-mediated immune response
- When an antigen is presented on a class I marker, the presenting cell will be destroyed (as it contains an intracellular threat)
Class II markers are only present on ‘professional' antigen presenting cells (monocytes) that engulf foreign pathogens via phagocytosis
- Class II markers present exogenous antigens (found externally) to helper T cells, which initiate a humoral immune response
- When an antigen is presented on a class II marker, the presenting cell is not destroyed (as the identified threat is extracellular)

Clonal Selection
The body contains millions of different lymphocytes that each recognise a single, particular antigen (the adaptive immunity is specific)
- Specific cytotoxic T lymphocytes are activated when antigens are presented on MHC class I markers (cell-mediated immunity)
- Specific helper T lymphocytes are activated when antigens are presented on MHC class II markers (humoral immunity)
Only one specific lymphocyte can be activated by a particular antigenic fragment to divide and form copies (clonal selection / expansion)
- However, a pathogen may contain multiple antigen fragments and hence activate multiple specific lymphocytes (polyclonal activation)

Cell Mediated Immunity
Cell-mediated immunity uses cytotoxic T cells to target endogenous antigens (i.e. intracellular pathogenic threats)
- Cancerous and virus-infected cells are not recognised as foreign and hence cannot be specifically targeted by antibodies
- These compromised cells present their antigenic fragments as a complex with their own self markers (MHC class I)
- Cytotoxic T cells can recognise these specific antigens and will release perforating enzymes when stimulated
- These enzymes cause the infected / cancerous cell to by lysed, preventing the further spread of infection
- NK cells function in a similar manner, but are non-specific (they respond to interferon secretion, not antigen presentation)

Humoral Immunity
Humoral immunity describes the pathway by which antibodies are produced by B lymphocytes to target exogenous antigens
- When monocytes engulf extracellular pathogens, they digest them within lysosomes to release antigenic fragments
- These fragments are presented on special surface receptors (MHC class II) that denote the material as being foreign
- The antigens are presented to helper T cells, which in turn secrete cytokines to activate the appropriate B lymphocyte
- Each B lymphocyte has a specific antibody on its surface that is capable of recognising a single specific antigen
- The appropriate B lymphocyte will divide and differentiate (via clonal selection) to form antibody-producing plasma cells
- The plasma cells are short-lived, but will mass produce the antigen-specific antibody for the duration of its circulating life span

Antibodies
An antibody (or immunoglobulin) is a protein produced by B lymphocytes (and plasma cells) that is specific to a given antigen
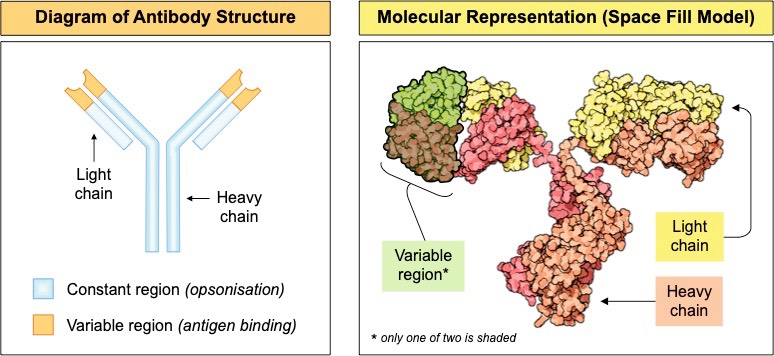
- Antibodies are made of 4 polypeptide chains that are joined together by disulphide bonds to form Y-shaped molecules
- The ends of the arms are where the antigen binds – these areas are called the variable regions and differ between antibodies
- The rest of the molecule is constant across all antibodies and serves as a recognition site for the immune system (opsonisation)
- Each type of antibody recognises a unique antigen, making antigen-antibody interactions specific (and part of adaptive immunity)
Antibody specificity is determined by the recombination of antibody genes in B lymphocytes (specifically the VDJ gene segments)
- Each B lymphocyte creates an antibody with a different variable region according to how the antibody genes are recombined
- Over 100 million specific antibodies are created by the body before an antigenic fragment has even been encountered
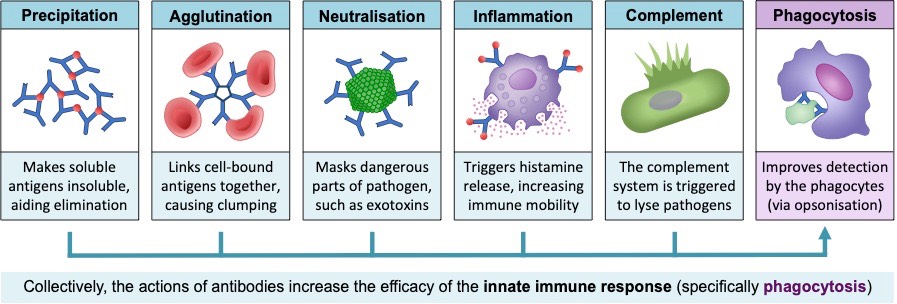
Antibodies aid in the destruction of pathogens by a number of different mechanisms:
- Precipitation – Antibody binding can cause soluble pathogens to become insoluble within the tissue fluid (precipitation)
- Agglutination – Antibodies can cause multiple pathogens to clump together, facilitating easier removal of the pathogens
- Neutralisation – Antibodies may occlude pathogenic regions, preventing the pathogen from binding to its target
- Inflammation – Antibodies may bind to mast cells, triggering an enhanced inflammatory response within the body
- Complement activation – Antibodies can trigger the activation of complement proteins (which can cause pathogen lysis)
Collectively, antibodies enhance the immune system by aiding the detection and removal of pathogens by phagocytic leukocytes
- The constant region of antibodies can be recognised by monocytes, improving pathogen identification (via opsonisation)
- The monocytes (principally macrophages) can now engulf and eliminate pathogens more efficiently, reducing disease symptoms
Immunological Memory
The adaptive immune system relies on the clonal expansion of B lymphocytes to produce sufficiently large numbers of antibodies
- This means there is a delay between the initial exposure to a pathogen and the production of large quantities of antibodies
- If pathogens can reproduce rapidly during this delay period, they can impede normal body functioning and cause disease
Memory cells are produced to prevent this delay in subsequent exposures and hence prevent disease symptoms developing
- When a B lymphocyte is activated and undergoes clonal expansion, a small proportion will differentiate into memory cells
- Memory B cells are long living and will survive in the body for many years, producing low levels of circulating antibodies
- If a second infection with the same pathogen occurs, memory B cells will react more vigorously to produce antibodies faster
- As antibodies are produced faster, the pathogen cannot reproduce in sufficient amounts to cause disease symptoms
- Hence, because pathogen exposure no longer causes the disease to occur, the individual is said to be immune
- Helper T cells and cytotoxic T cells also produce memory cells to aid in the development of ongoing immunity
