Key Knowledge:
|
The rate of enzyme catalysis can be increased by improving the frequency of successful collisions via:
- Increasing the molecular motion of the particles (thermal energy can be introduced to increase kinetic energy)
- Increasing the concentration of particles (either by increasing the substrate or enzyme concentrations)
- Maintaining enzyme-substrate specificity (enzyme denaturation will change the conformation of the active site)
Factors Affecting Enzyme Activity
The three main conditions that may affect the rate of an enzyme-catalysed reaction are temperature, pH and substrate concentration
Temperature
- Low temperatures result in insufficient thermal energy for the activation of an enzyme-catalysed reaction to proceed
- Increasing the temperature will increase the speed and motion of both enzyme and substrate, resulting in higher enzyme activity
- This is because a higher kinetic energy will result in more frequent successful collisions between the enzymes and substrates
- At an optimal temperature (which may vary for different enzymes), the rate of enzyme activity will be at its peak (highest level)
- Higher temperatures will cause enzyme stability to decrease, as the thermal energy disrupts the enzyme’s hydrogen bonds
- This causes the enzyme (particularly the active site) to lose its shape, resulting in the loss of activity (as denaturation occurs)

pH
- Changing the pH will alter the charge of the enzyme, which in turn will alter protein solubility and overall enzyme shape
- Changing the shape or charge of the active site will diminish its ability to bind the substrate, abrogating enzyme function
- Enzymes have an optimal pH (may differ between enzymes) and moving outside this range diminishes enzyme activity

Substrate Concentration
- Increasing the substrate concentration will increase the activity of a corresponding enzyme
- More substrates mean there is an increased chance of enzyme and substrate colliding and reacting within a given period
- After a certain point, the rate of activity will cease to rise regardless of any further increases in substrate levels
- This is because the environment is saturated with substrate and all enzymes are bound and reacting (Vmax)

Limiting Factors
When a chemical process depends on multiple conditions, the reaction rate will be limited by the factor nearest to its minimum value
- This is known as the law of limiting factors (i.e. the factor in the shortest supply will determine the rate of the biochemical process)
In complex biochemical pathways, the initial inputs (reactants) affect overall reaction rates in a manner akin to substrate concentration
- Glucose is an initial input for the process of cell respiration, while carbon dioxide is an initial input for the process of photosynthesis
- Oxygen is an initial input for aerobic respiration only and so will not directly impact the rate of anaerobic respiration or fermentation
- Water availability may be hard to assess for photosynthesis, as water can be produced by cell respiration or condensation reactions
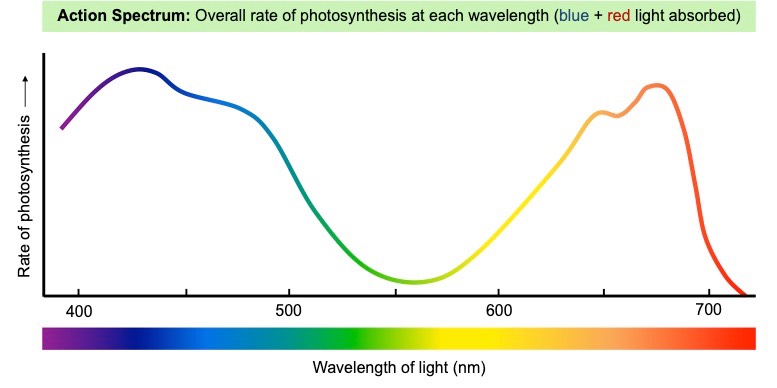
- Light intensity influences the photo-activation of chlorophyll, and different wavelengths have different effects (green light is reflected)
Enzyme Inhibitors
An enzyme inhibitor is a molecule that disrupts the normal reaction pathway between an enzyme and a substrate
- Enzyme inhibitors can be either competitive or non-competitive depending on their mechanism of action
Inhibition of enzymes may be either reversible or irreversible depending on the specific effect of the inhibitor being used
- Irreversible inhibitors form strong covalent bonds with the enzyme in order to form a permanent attachment
- Reversible inhibitors form weaker, temporary attachments with the enzyme and thus can potentially be dissociated
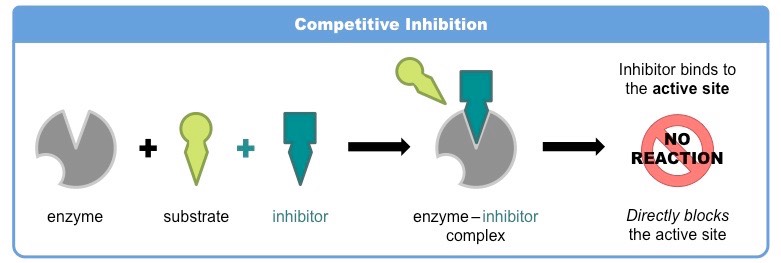
Competitive Inhibition
- Competitive inhibition involves a molecule, other than the substrate, binding to the enzyme’s active site
- The molecule (inhibitor) is structurally and chemically similar to the substrate (hence able to bind to the active site)
- The competitive inhibitor blocks the active site and thus prevents substrate binding
- As the inhibitor is in competition with the substrate, its effects can be reduced by increasing substrate concentration
Non-competitive Inhibition
- Non-competitive inhibition involves a molecule binding to a site other than the active site (an allosteric site)
- The binding of the inhibitor to the allosteric site causes a conformational change to the enzyme’s active site
- As a result of this change, the active site and substrate no longer share specificity, meaning the substrate cannot bind
- As the inhibitor is not in direct competition with the substrate, increasing substrate levels cannot mitigate the inhibitor’s effect
