Key Knowledge:
|
Gene cloning is the process of isolating and copying a gene of interest in order to facilitate its expression within a host organism
Gene cloning occurs over three key stages:
- Isolation of a gene of interest and a vector (gene delivery vehicle)
- Insertion of the gene of interest into the vector (via digestion and ligation)
- Integration of the recombinant vector into a host cell (and subsequent gene expression)
Step 1: Isolating Gene and Vector
A gene is a sequence of DNA that encodes a specific characteristic (via the synthesis of proteins)
- DNA can be isolated from cells by centrifugation (heavier components – such as nuclei – are separated) and then precipitation
- The DNA can then be amplified via the polymerase chain reaction (using primers that specifically target the gene sequence)
- Gene sequences can also be generated from mRNA using reverse transcriptase – these DNA sequences (cDNA) lack introns
A vector is a molecule that is used as a vehicle to carry the gene of interest into a foreign cell
- Bacterial plasmids are commonly used as vectors because they are capable of autonomous self-replication and expression
- These plasmids may be modified for further functionality (e.g. selection markers, reporter genes, inducible expression promoters)
- Other types of vectors include modified viruses (e.g. bacteriophages) and artificial chromosomes
Common Features of a Typical Plasmid Vector

Step 2: Combining Gene and Vector
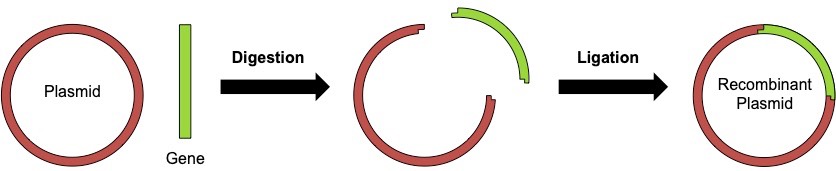
The incorporation the gene of interest into the plasmid vector involves two key processes:
- Digestion: Both the gene of interest and plasmid are cut with specific endonucleases (restriction enzymes)
- Ligation: The digested gene of interest and plasmid are then fused together to form a recombinant plasmid
In digestion, both a gene of interest and vector cut with restriction enzymes at specific recognition sites
- Restriction enzymes cleave the sugar-phosphate backbone to generate blunt ends or sticky ends (complementary overhangs)
- Scientists will often cleave the vector and gene with two different ‘sticky end’ restriction endonucleases (double digestion) to ensure the gene is inserted in the correct orientation and to prevent the vector from re-annealing without the desired insert
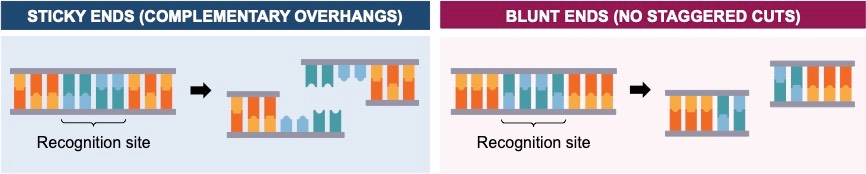
In ligation, the gene of interest is inserted into a plasmid vector that has been cut with restriction endonucleases
- If digestion produces sticky ends, then the ends must be complementary (i.e. cut with the same enzyme) for ligation to occur
- The gene and vector are spliced together by the enzyme DNA ligase to form a recombinant plasmid
- DNA ligase joins the vector and gene by fusing their sugar-phosphate backbones together with a covalent phosphodiester bond
- The recombinant plasmid can be separated and isolated from the standard plasmid (no gene included) using gel electrophoresis
Step 3: Integrating Recombinant Vector into Host Cell
The recombinant construct (including the gene of interest) is finally introduced into an appropriate host cell or organism
- This process is called transfection (for eukaryotic cells), transformation (for bacterial cells) or transduction (if a viral vector is used)
- A range of methods may be used to introduce the recombinant construct, including heat shocking, electroporation or liposomes
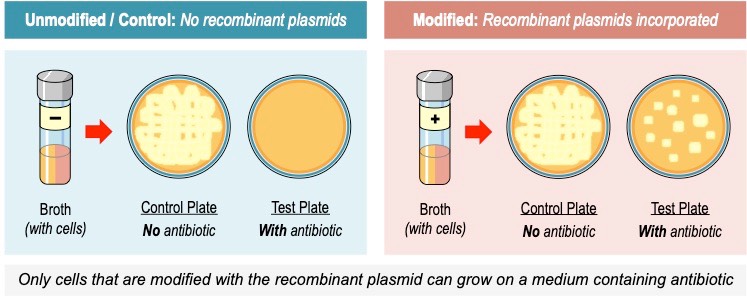
Antibiotic selection is commonly used in order to identify which cells have successfully incorporated the recombinant construct
- An antibiotic is a compound that specifically targets prokaryotic features (and hence kills or inhibits bacterial cell growth)
- The plasmid vector contains an antibiotic resistance gene, so only transgenic cells will grow in the presence of antibiotic
- Antibiotic selection can also be used to successfully select for transgenic eukaryotic cells if the antibiotic targets the prokaryotic features of the chloroplast or mitochondria (these structures were once prokaryotes, but became organelles via endosymbiosis)
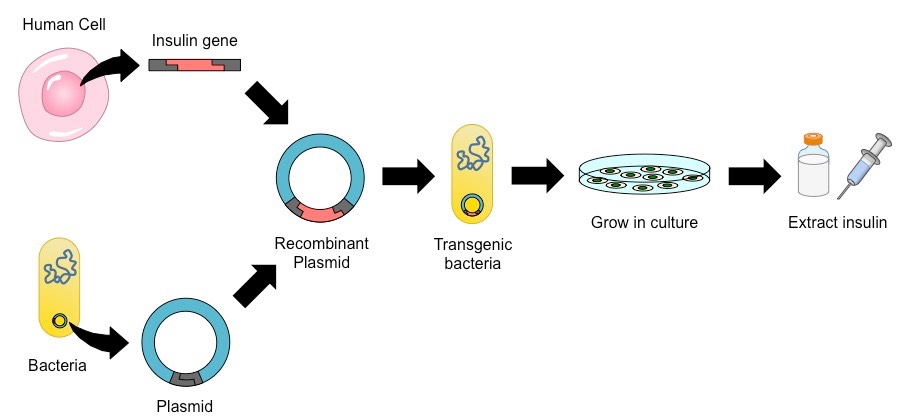
Application: Production of Human Insulin
The ability to transfer genes between species has been utilised to produce human insulin in bacteria (for mass production)
- Because the genetic code is universal, human genes can be expressed by bacterial cells provided introns are removed
- Reverse transcriptase can be used to create a cDNA copy of the human insulin gene without any intron sequences
The benefit of using transgenic bacteria to produce human insulin is that bacteria can divide and reproduce very rapidly
- When the bacteria is cultured in a fermentation tank, large quantities of human insulin can be produced in short periods of time
- Once produced, the human insulin is purified and packaged for human use (to treat individuals with type II diabetes mellitus)