Key Knowledge:
|
A catalyst is a substance that enables a chemical reaction to proceed at a faster rate or under different conditions (e.g. lower temperature)
- Catalysts lower the amount of energy required for a reaction to proceed (activation energy), allowing a reaction to occur more readily
- Catalysts are not changed or consumed by the reactions they influence and so occur at relatively low levels and can be re-used
Enzymes
Enzymes are globular proteins which act as biological catalysts and speed up the rate of a reaction by lowering the activation energy
- Every enzyme will only react with specific molecule called a substrate, which binds to a region of the enzyme called the active site
- The active site and substrate will complement each other in terms of both shape and chemical properties (e.g. opposite charges)
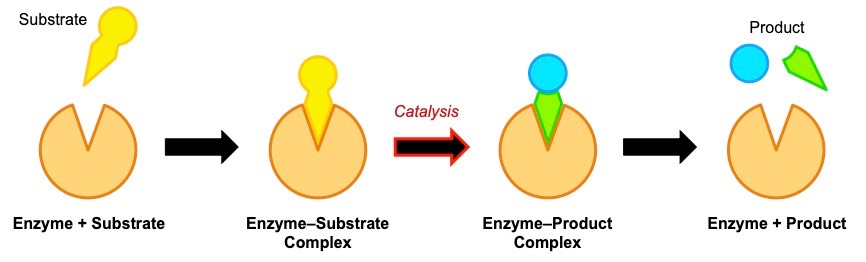
When a substrate binds to the active site, an enzyme-substrate complex is formed (as per the ‘lock and key’ model)
- The active site is not completely rigid however and may undergo a conformational change in shape to better fit the substrate
- This conformational change may stress and destabilise the bonds in the substrate, hence lowering the activation energy
- When the substrate has been converted into a product, it will dissociate from the enzyme (allowing the enzyme to be re-used)
Enzyme reactions typically occur in aqueous solutions as these reflect the internal conditions of an organism (cytoplasm, interstitial fluid)
- Consequently, the substrate and enzyme are usually moving randomly within the solution (Brownian motion) until a successful collision
- Sometimes an enzyme may be fixed in position (e.g. if it is membrane-bound) – this will serve to localise the reaction to a particular site
Coenzymes
A coenzyme is a complex organic molecule that is required for an enzyme’s metabolic activity (it assists with the catalysis of a reaction)
- Coenzymes cycle between two states: a loaded form that can be used and an unloaded form (similar to a charged or expended battery)
- Examples of biologically significant coenzymes include ATP (transfers energy) and hydrogen carriers (transfers protons and electrons)
Adenosine Triphosphate (ATP)
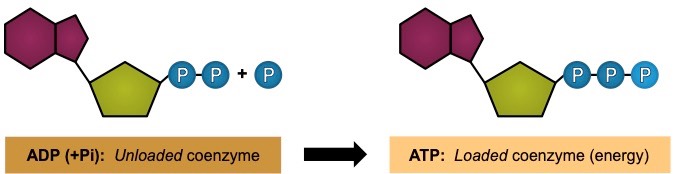
ATP (adenosine triphosphate) is a high energy molecule that functions as an immediate source of power for cell processes
- One molecule of ATP contains three covalently linked phosphate groups – which store potential energy in their bonds
- When ATP is hydrolysed (to form ADP + Pi) the energy stored in the phophate bond is released to be used by the cell
Hydrogen Carriers
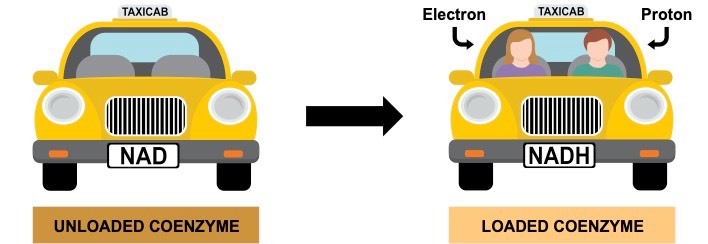
Hydrogen carriers are coenzymes that transport protons and electrons between chemical reactions (functions like a chemical taxi)
- Hydrogen carriers are loaded by oxidation reactions (become reduced), and unloaded in reduction reactions (become oxidised)
- The protons and electrons can be used to help synthesise organic macromolecules via anabolic reactions (e.g. photosynthesis)
- Hydrogen carriers also function as intermediate energy sources (via their energised electrons) and can be used to make ATP
- Examples of hydrogen carriers include NADH and FADH2 (used by mitochondria), as well as NADPH (used by the chloroplast)